By DR. Martin Lukindu
The recent freeze on certain U.S. global aid programs—spanning health, environment, refugee support, and other key sectors—has triggered widespread concern, particularly in Uganda. Over 1.8 million refugees and their host communities in Uganda are facing acute shortages of food, medicine, and basic services, as vital international support hangs in the balance.
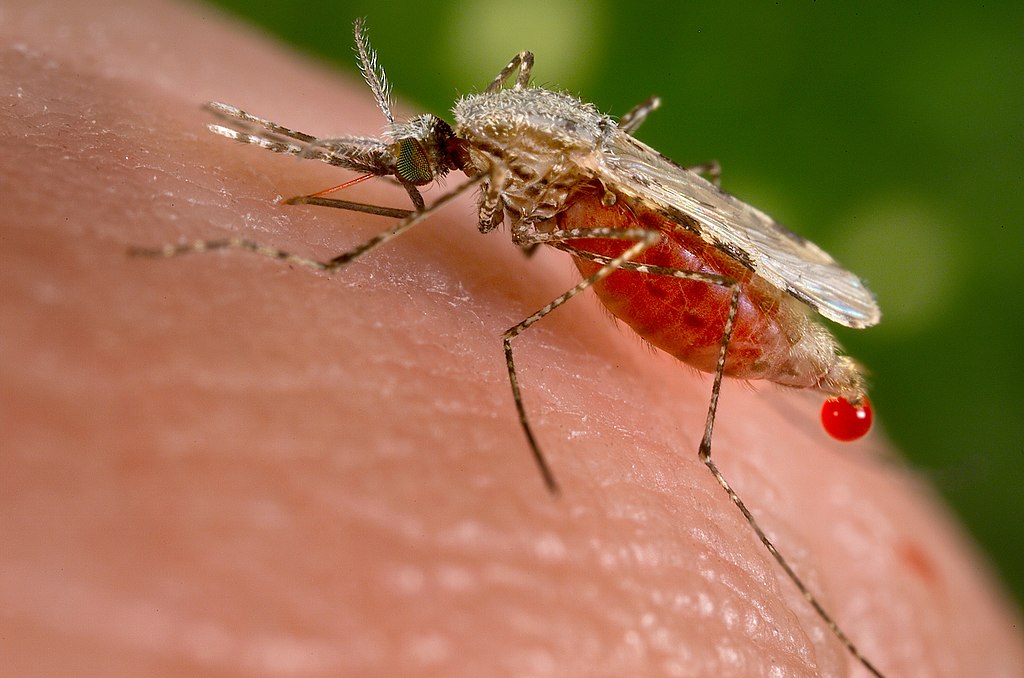
The timing couldn’t be worse. Uganda is entering its annual short rainy season, which historically brings a surge in malaria cases. Compounding this crisis are emerging challenges such as partial drug resistance to anti-malaria drugs and resistance to insecticides used in vector control interventions.
But amid the uncertainty, there is a ray of hope: Uganda has rolled out a malaria vaccine this April, targeting children aged six months to five years. Experts are optimistic that the program could save countless young lives and marks a significant milestone in the fight against malaria.
Still, experts warn against over-reliance on any single intervention—or donor for malaria control. Consequently, as Uganda navigates these pressing challenges, the need for sustained diverse investment in current control measures while exploring bold innovative solutions has never been clearer.
Malaria: an ever-evolving threat requiring novel tools
As scientists race to develop more effective drugs and insecticides in the battle against malaria, mosquitoes are evolving just as quickly to adapt to any new interventions. In Uganda malaria is transmitted primarily by Anopheles gambiae, Anopheles arabiensis, and Anopheles funestus—species well-established across the country.
But now, a new threat is emerging on the horizon. Anopheles stephensi, an invasive proficient malaria vector originally found in South Asia, is steadily making its way across the African continent. First detected in Djibouti in 2012, the species has since spread to Ethiopia, Sudan, Somalia, Kenya, and Nigeria, raising alarms among health experts.
What sets Anophensi stephensi apart from native vectors is its ability to uniquely adapt to urban environments, breeding in common household containers such as water tanks and jerrycans, and thriving in high humidity and temperatures. Its adaptability makes it a particularly insidious threat to African cities that have traditionally seen lower malaria transmission rates.
Even more concerning, An. stephensi is known for its resistance to many insecticides, making it harder to control through conventional methods. Indeed, in countries where it has already established itself, the result has been sharp increases in malaria cases.
So far, Anopheles stephensi has not yet been detected in Uganda, although surveillance and public awareness campaigns are being scaled up in an effort to prevent its entry and slow its spread.
While invasive species like Anopheles stephensi present new threats, scientists in Uganda and beyond are turning to cutting-edge genetics to outsmart the mosquito. For years, researchers have explored ways to interrupt mosquito reproduction in order to break the malaria transmission cycle.
Traditional genetic modification techniques, aimed at reducing mosquito populations by altering reproductive genes, have shown promise. However, such modifications only have a 50% chance of being inherited, meaning the changes can fade over generations unless costly and repeated releases of modified mosquitoes are made.
Now, a global research consortium known as Target Malaria is developing a groundbreaking approach using gene drive technology, based on the precision gene-editing tool CRISPR. With gene drive, beneficial genetic changes are passed on to nearly all offspring, allowing the modification to spread quickly and sustainably through mosquito populations.
Target Malaria—operating in Uganda at the Uganda Virus Research Institute (UVRI), as well as in Burkina Faso, Italy, the United States, and the United Kingdom—is exploring two key strategies: one, to impair the egg-laying capacity of female mosquitoes, and two, to bias mosquito reproduction so more males are born than females. Since only female mosquitoes bite and lay eggs, reducing their numbers would have a direct impact on malaria transmission.
As part of this effort, the UVRI team in Entebbe is currently studying non-gene drive mosquitoes genetically skewed to produce more male offspring. These were imported from the U.S. Centers for Disease Control (CDC) in Atlanta in May 2024, following all necessary regulatory approvals and community engagement processes.
The mosquitoes are being studied in a secure Arthropod Containment Level 2 (ACL-2) insectary, ensuring safe, controlled research. Researchers hope these early studies will pave the way for the future use of gene drive mosquitoes in Africa’s malaria-endemic regions.
With innovative science on one hand and looming threats on the other, the fight against malaria continues—more urgent, more complex, but also more hopeful than ever.
Written by DR. Martin Lukindu -Post-Doctoral Research Fellow -Target Malaria
The Author joined Target Malaria in 2013 as a field entomology coordinator, and left in 2015 for his doctoral studies. He rejoined the project in 2021 as a post-doctoral research associate. He is using the close-kin marker-recapture (CKMR) approach to understand the dispersal ranges, fecundity and other aspects of the vector biology of isolated Anopheles gambiae populations in lacustrine islands of Lake Victoria in Uganda. His research is important in guiding potential confined releases of gene drives against malaria vectors in geographically/genetically isolated islands.

He previously worked as a research assistant in the department of entomology at UVRI in which he assessed population genetics, oviposition behaviors and seasonal population dynamics of arboviral vector Aedes africanus in Uganda.
Martin holds a PhD in Biological Sciences from the University of Notre Dame, USA, a BSc degree and an MSc in Zoology/Entomology from Makerere University, Uganda. His doctoral dissertation was about molecular tools and genetic approaches for studying the genetic structure of major malaria vectors in Sub-Saharan Africa.
About Target Malaria:
Target Malaria is a not-for-profit international research consortium with partners in Africa, Europe and North America that aims to develop and share new, cost-effective and sustainable genetic technologies to modify mosquitoes and reduce malaria transmission. Our vision is to contribute to a world free of malaria. We aim to achieve excellence in all areas of our work, creating a path for responsible research and development of genetic technologies.
Target Malaria receives core funding from the Bill & Melinda Gates Foundation and Open Philanthropy. The lead grantee organisation is Imperial College London.








The recent freeze on U.S. global aid programs is deeply troubling, especially in Uganda, where the impact on refugees and host communities is devastating. The combination of food and medicine shortages, alongside the seasonal malaria surge, creates a perfect storm of hardship. While the introduction of the malaria vaccine is a hopeful step, it’s concerning to see how dependent Uganda is on external support. The research on genetically modified mosquitoes is fascinating, but the 50% inheritance rate raises questions about its long-term viability. Wouldn’t it be more effective to focus on multiple, sustainable solutions rather than relying on costly, one-time interventions? How do you see Uganda balancing innovation with immediate needs in this crisis? It’s a delicate situation, and the world should be paying closer attention. Thoughts?